Background: Hemophagocytic lymphohistiocytosis (HLH) is a rare, life-threatening, hyperinflammatory syndrome caused by the overproduction of proinflammatory cytokines, such as interferon gamma (IFNγ). Secondary HLH (sHLH) occurs in the context of an underlying disease (malignancy, rheumatologic, or metabolic disease) and/or an infection. Emapalumab is a fully human anti-IFNγ monoclonal antibody approved by the FDA in 2018 for refractory, recurrent, or progressive primary HLH (pHLH), or intolerance with conventional therapy. Since its approval, there are limited data on emapalumab use in a real-world setting. The REAL-HLH study assessed real-world treatment patterns and outcomes among patients with HLH treated with emapalumab.
Methods: A retrospective medical chart review conducted across 33 US hospitals identified patients treated with ≥1 dose of emapalumab between November 20, 2018, and October 31, 2021. Data were extracted from time of emapalumab initiation to end of data availability, death, or study end (December 31, 2021). Results are presented for the subgroup of patients with sHLH treated off-label with emapalumab.
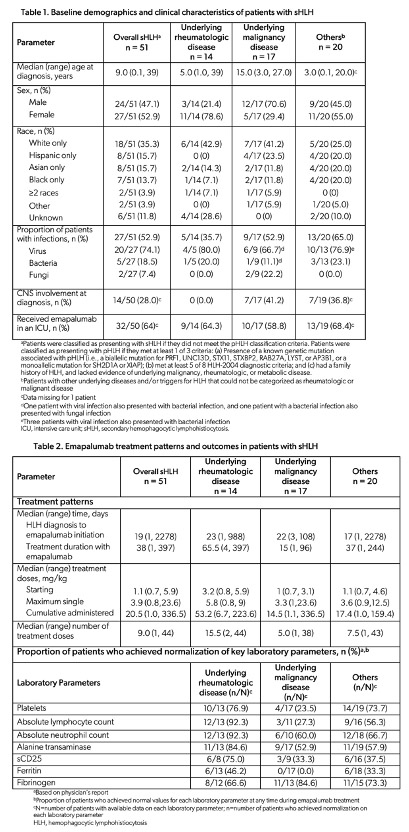
Results: Overall, 105 patients were included in this chart review of which 98 (93.3%) were reportedly treated for HLH-related conditions. Of these, 51/98 (52%) did not meet the pHLH classification criteria (Table 1) and were categorized as presenting with sHLH. These patients (n=51) were grouped based on the underlying disease (i.e., rheumatologic, n=14; malignancy, n=17; and others, n=20). The “others” category comprised a miscellaneous group of patients with underlying diseases and/or HLH triggers that could not be categorized as rheumatologic or malignant disease. Median (range) age at sHLH diagnosis was 9 (0.1-39) years. Baseline demographics and clinical characteristics are presented in Table 1. More than half (32/50; 64%) of these patients were critically ill and received emapalumab in an intensive care unit. Emapalumab was primarily initiated to treat refractory (20/51; 39.2%), progressive (15/51; 29.4%), or recurrent (8/51; 15.7%) disease. The median (range) time from sHLH diagnosis to emapalumab initiation and emapalumab treatment duration are presented in Table 2. Median (range) starting, maximum single, cumulative, and number of administered doses of emapalumab are presented in Table 2. Among patients who achieved normalization of key laboratory parameters (i.e., platelets, absolute lymphocyte count, absolute neutrophil count, alanine transaminase, sCD25, ferritin, and fibrinogen [ Table 2]), the median time to first normalization of all these laboratory parameters ranged from 7-38.5 days for those with underlying rheumatologic disease; 7-38 days for those with underlying malignancy; and 7-11 days among patients in the “others” category. The 12-month survival probability following emapalumab initiation was 85.7% for patients with underlying rheumatologic disease, 23.5% for those with underlying malignancy, and 55% for patients in the “others” category.
Conclusion: This is the first study to report real-world treatment patterns with emapalumab across a diverse patient population with sHLH. A phase 3 clinical trial of emapalumab in patients with sHLH/macrophage activating syndrome (MAS) and underlying rheumatologic disease is ongoing (NCT05001737).
OffLabel Disclosure:
Allen:Sobi, Inc: Consultancy. Chandrakasan:SOBI: Consultancy, Honoraria; Pharming: Honoraria. Jordan:Sobi: Consultancy, Research Funding. Leiding:Sobi, Inc: Consultancy; bluebird bio: Current Employment. Oladapo:Sobi, Inc: Current Employment. Pednekar:Sobi, Inc: Consultancy. Walkovich:X4 Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; St. Jude: Honoraria; Pharming: Membership on an entity's Board of Directors or advisory committees; UpToDate: Patents & Royalties: Royalties from UpToDate; American Society of Hematology: Honoraria; Horizon: Membership on an entity's Board of Directors or advisory committees; NICER: Other: NICER Consortium, Executive Chair ; Sobi: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Yee:Sobi, Inc: Ended employment in the past 24 months.
Emapalumab is approved for patients with refractory, recurrent, or progressive primary HLH (pHLH), or intolerance with conventional therapy

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal